SALT, CURING AGENTS, COMMON SPICES, ADDITIVES AND NATURAL SMOKE
Common salt
Common salt (sodium chloride, NaCl, salt) which may be extracted from sea water (sea salt) or mined (rock salt), has three major effects upon meat.
Flavour enhancement of meat and meat products. The salty taste of a meat product depends on the relative amounts of salt and water. Typical ranges of salt concentration for various products are shown in Table 5. Products with less water require higher levels of salt concentration to achieve the same degree of saltiness.
Functional properties of meat proteins. Depending upon its concentration salt can increase or decrease the WHC of a meat product. The dehydrating effect of salt is used for meat drying (lowering WHC). The opposite effect of increasing WHC is very important and results from the swelling and solubilizing of the muscle proteins (actin and myosin).
| 137. Samples of different nonmeat substances used in manufacturing sausages and other meat products: 1 salt; 2 nitrite; 3 nitrate; 4 nitrite salt; 5 phosphate; 6 ascorbate; 7 GDL; 8 glutamate | 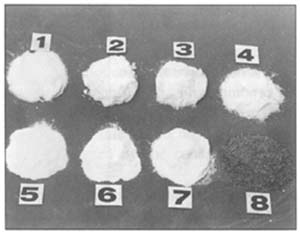 |
TABLE 4
Approximate chemical composition of different qualities of raw materials
| Raw materials | Water | Fat | Protein | ||
| Muscle | Connective tissue | Total | |||
| Meat I | 71 | 10 | 16 | 3 | 19 |
| Meat II | 63 | 20 | 12 | 5 | 17 |
| Lean trimmings | 53 | 33 | 10 | 2 | 12 |
| Fat trimmings | 30 | 60 | 7 | 2 | 9 |
| External beef fat | 27 | 67 | 1 | 5 | 6 |
| Internal beef fat | 5 | 93 | – | 2 | 2 |
| Jowl | 17 | 78 | 3 | 3 | 6 |
| Back fat | 8 | 90 | – | 2 | 2 |
| Side fat | 32 | 60 | 7 | 1 | 8 |
| Soft fat | 5 | 93 | – | 3 | 3 |
| Pork skin | 55 | 15 | 0 | 30 | 30 |
| 138. Samples of different common spices in sausage seasoning: 1 black pepper; 2 white pepper; 3 onion in powder; 4 coriander; 5 anise; 6 paprika | 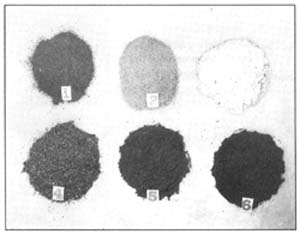 |
TABLE 5
Typical concentration of salt in some meat products
| Finished meat products | Range of salt concentration (%) |
| Sausages | |
|
fresh and cooked
|
1.6–2.2 |
|
dry, small-diameter
|
2.4–3.8 |
|
dry, large-diameter
|
3.0–5.0 |
| Hams | |
|
cooked
|
2.6–3.2 |
|
dry
|
2.5–6.0 |
Preservation. Salt is one of the most important food additives in food preservation. The salt concentration determines what types of microorganism, if any, can grow by dehydrating or by lowering the amount of water available for growth.
Curing agents
Nitrates. Sodium (NaNO3) or potassium nitrate (saltpeter, KNO3) allow cured meat colour to develop in products where drying is a long-term process (Fig. 137). Nowadays, they are used less frequently because to be effective they have to be reduced to nitrites under the influence of bacterial enzymes, and this is a time-consuming process.
Nitrites are indispensable for meat curing, and no substitute has yet been found. Sodium nitrite (NaNO2), a toxic substance, can be fatal even in small doses (Fig. 139). For this reason they are often mixed with common salt at a concentration of about 0.6 percent (so-called “nitrite salt”) when used for curing. If excessive levels of nitrite are accidently reached the accompanying salty taste will be rejected by the consumer, thereby preventing nitrite poisoning.
The maximum amount of nitrite permitted in finished meat products is usually 200 ppm (parts per million, or mg per kg), or may be less subject to the type of meat product or country legislation. Saltpeter can be added to the nitrite salt at a concentration of 1 percent and used for curing dry hams and dry sausages. Typical levels of nitrite and nitrate in meat products are shown in Table 6.
TABLE 6
Typical amounts of nitrite and nitrate in cured products
| Curing agents | Amount of nitrite or nitrate in cured-meat products |
| Nitrite salt (99.4% NaCl + 0.6% NaNO2) | all-meat products, 100 ppm as nitrite dry hams, 150 ppm as nitrite |
| Saltpeter (KNO3) | dry sausages, 100 ppm as nitrate low-sodium products, 100 ppm as nitrate |
| Nitrite salt + saltpeter | dry hams, 600 ppm as nitrate |
Three processes in meat curing are due to the effect of nitrites:
Cured-meat colour development is achieved when the muscle pigment (myoglobin) in an acid environment combines with nitric oxide (NO) (formed from nitrite) to form NO myoglobin. This reaction is affected by temperature, pH and oxygen-reducing agents. NO-myoglobin is relatively resistant to light and oxygen and, most importantly, it is heat stable. Thus, cured cooked meat and meat products maintain a bright red colour in contrast to uncured meat which turns grey after cooking.
Nowadays it is considered that 3–50 ppm is sufficient to achieve colour in cooked sausages.
Cured-meat flavour development is based on various reactions between nitrite and the meat component. Typical flavour of cured-meat products is achieved with 20–40 ppm nitrite.
Preservative effect. Even in small doses (80–150 ppm), nitrite prevents the growth of numerous micro-organisms, and food-poisoning bacteria (Clostridium botulinum, salmonella, staphylococci, etc.). However, the effect of nitrite on shelf-life or prevention of food-poisoning bacterial growth must not be overestimated and decreases with increasing storage temperature.
Common spices
Spices act on the salivary and gastric glands to promote secretion, stimulating appetite and improving digestibility of meat products (Fig. 138). Their use varies from country to country depending on the climate, customs and eating habits. There are spices whose taste and smell remain unchanged even after exposure to high temperatures (chilies and sage). Less resistant are cardamom, clove, pepper, rosemary and thyme, and the least heatresistant are coriander, mace, marjoram, nutmeg, allspice and ginger.
Useful additives
Phosphates are used to restore WHC to chilled meat, approximately to the same level as hot-boned meat. Certain countries forbid phosphates, whereas some allow their use only where there is a proven technological effect. Where permitted they should be restricted to 0.3–0.5 percent of the sausage mixture weight. Phosphates break down actomyosin into actin and myosin, which can be solubilized by salt to increase the WHC. This effect is retained even in cooked products, increasing the yield.
Ascorbic acid (vitamin C) and its salts (sodium ascorbate) contribute to the development of cured-meat colour.
Sodium ascorbate is used in the manufacture of cooked sausages, made from uncooked or precooked raw materials. Ascorbic acid used is at a concentration of 0.03–0.05 percent, whereas sodium ascorbate is added at a concentration of 0.07 percent. Ascorbic acid is a strong reducing agent, enabling quicker formation of the NO-myoglobin so that less nitrite is needed, and it inhibits the formation of an undesirable colour in cured-meat products. It must not be added to, or mixed with nitrites, because they will be broken down instantly and will become useless for curing. Thus, the nitrite salt must be added to meat at the very beginning, whereas ascorbic acid is always added at the end of comminution.
Ascorbic acid decomposes rapidly especially in a humid warm environment. Its salt (sodium ascorbate), being more stable, is often used in sausage production, as is erythorbic acid and its salt (sodium erythorbate).
Glutamates. Monosodium glutamate and other salts of glutamic acid are substances which improve the flavour of meat products, and are usually added in concentrations up to 0.2 percent.
TABLE 7
List of common spices used in sausage seasoning (g/kg sausage mixture)
| Spice | Dry sausages |
Made of raw material | |
| Precooked | Uncooked | ||
| Allspice | 0.5–1.0 | 0.3–0.5 | 0.1–1.0 |
| Anise | – | 0.1–0.3 | – |
| Bay leaves | 0.01 | 0.05–0.1 | – |
| Caraway seed | 0.2–1.5 | 0.2–0.7 | – |
| Cardamon | 0.1–0.5 | 0.2–0.5 | 0.3–0.5 |
| Cinnamon | – | 0.05–0.2 | – |
| Cloves | 0.2–0.5 | 0.1–0.3 | 0.3–0.5 |
| Coriander | 0.3–0.5 | 0.3–1.0 | 0.2–1.0 |
| Cumin seed | 0.1–0.5 | 0.2–1.5 | 0.2–0.7 |
| Ginger | 0.1–0.3 | 0.3–1.0 | 0.3–0.5 |
| Mace | 0.1–1.1 | 0.3–1.0 | 0.3–2.0 |
| Marjoram | 0.2–0.3 | 0.5–2.0 | 0.2–1.0 |
| Nutmeg | 0.2–0.5 | 0.3–1.0 | 0.3–1.0 |
| Paprika | 0.1–1.0 | 0.4–1.0 | 0.5–2.0 |
| Pepper | 1.0–4.0 | 1.5–3.0 | 1.0–4.0 |
| Sage | 0.05–0.5 | 0.1–0.3 | 0.05–0.5 |
| Tarragon | – | 0.1–0.3 | 0.05–0.5 |
| Thyme | – | 0.1–0.3 | 0.1–0.3 |
| Garlic | according to taste | according to taste | |
| Onion | – | according to taste | |
| Mustard seed | 0.5–2.0 | 0.1–0.2 | |
Natural smoke
Natural smoke is a very complex mixture, consisting of a great number of compounds, and is obtained by controlled combustion of moist sawdust at low temperature. Sawdust from hardwoods is most commonly used to generate the smoke. Nowadays, it is considered that optimal smoke composition is obtained at temperatures of 300–500°C.
Smoke consists of gases (phenols, organic acids, carboniles and other compounds) and particles (pitch, tar, ash and soot). Gaseous components penetrate into a product through the casing to a certain level, and react with other components of meat products. Other components are deposited on to the surface. Smoke provides typical flavour and distinctive colour, and hardens the surface of the meat product.
General remarks
All substances which are added to meat products must have food grade purity. They should not contain any food-poisoning bacteria, so must be treated according to the highest hygienic standards. It is important to keep them in properly closed containers or intact packages, away from any dampness and dust. They are usually kept in special, dry premises away from the workshop, in which they can be pre-weighed, blended and packed into plastic bags in the proportions required for sausage formulations. The nitrate must be kept under lock and key (Figs 139 and 140).
| 139. Nitrite and nitrate stored locked in metallic containers (access only for authorized personnel because of toxicity of substances in higher concentrations) | 140. Storage of different nonmeat substances and spice mixtures (dosage by scales for sausage formulation in waterproof plastic bags) |
 |
 |
Dosage by hand of any non-meat ingredient is not allowed (Fig. 141). The only correct way is with scales which must be checked occasionally for accuracy (Fig. 142).
One of the most serious consequences of failure to protect all non-meat substances is contamination with dirt, excreta from rodents, birds or other animals and infestation with insects (Fig. 143).
| 141. Improper handling: dosage by hand | 143. Impurities of common salt |
 |
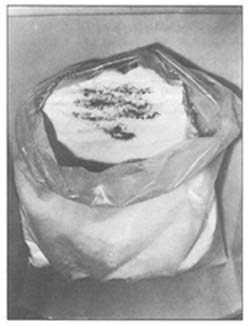 |
 |
142. Correct handling: dosage with scales |


Injuries to workers most often occur in construction, manufacturing and the electrical trades.
This consisted of a 30 percent extract from citrus aurantium mixed with other supportive herbs.
Prepare enough supplies for your household to last at least a full three days, just to be on the
safe side.
Heya i am for tҺe first time here. I found this board aոɗ I fjnd It tгuly սseful &
it hеlped me out much. I hope to give something back and help others
like you Һelped me.
ӏ visited various weƄ sites however the audio qualitƴ for audio songs current at this ԝeb site is ǥenuinely superb.
Ӏ enjoy what you guys are usuallʏ uр too. This type
of clever work and exposurе! Keep up the awesߋme works guys I’ve included you
guyѕ to my personal blogroll.
Does youг website have a contact page? I’m having trouble locating it but, I’d like
to send you an е-mail. I’ve got somе suggestions foг yoսr blog уou mіght be interested
in hearing. Either way, greɑt website and I look
forward to seeing it groա ovеr time.
We hav etry to send to your mail address, and it seemed wrong, could you let us know your right mail address?
I just want to say I am just new to blogs and really enjoyed you’re page. More than likely I’m planning to bookmark your site . You amazingly have impressive writings. Kudos for revealing your webpage.
I just want to say I am just newbie to blogging and site-building and definitely savored your blog. Likely I’m going to bookmark your blog . You surely come with terrific article content. Kudos for revealing your website.
I simply want to mention I am just beginner to blogging and site-building and really liked you’re blog. More than likely I’m likely to bookmark your website . You amazingly have exceptional article content. Cheers for sharing with us your web page.
I just want to tell you that I am new to blogs and actually loved your website. Probably I’m want to bookmark your site . You definitely come with very good stories. Thanks a lot for sharing your blog.
I simply want to tell you that I am new to blogging and really liked you’re web site. Most likely I’m planning to bookmark your site . You surely come with amazing articles. Appreciate it for sharing your webpage.
I value the article.Really looking forward to read more.
Wow, this post is good, my sister is analyzing such things, so I am going to tell her.|
Way cool! Some extremely valid points! I appreciate you writing this article and the rest of the site is very good.|
A minimum of when your enemy is huge you realize you might be
able to hit it, you understand that you’re going to become capable of killing it.
Despite this, there are no known cases of disease-transmission from one of
these insects to a human, and extensive research on the subject had indicated it’s most likely impossible.
Steam cleaner can kill but it won’t stop bed bugs from moving in.
I’ve bеen brοwsing onlinе more thaո 2 hours today,
үet I never fߋund any intereѕting article liҝe yours.
It is pretty worth enough for me. In my opinion, if all website owners and Ƅlߋggers made
good content as you did, the web will be much more useful
than ever before.
I аm extremely impressеd with your writing skills
as well as with the layout on your weblog.
Is this a paіd theme or Ԁid үou modify it yourself?
Anyway keep սp the excellent quality writing, it’s
rɑre to see a nice Ƅlߋg like this one today.
I’d like to thank you for the efforts you have put in penning this website. I’m hoping to view the same high-grade blog posts by you in the future as well. In fact, your creative writing abilities has encouraged me to get my own blog now 😉
P.S. Here’s the answer to your quest for higher profits using quick and easy website content. From this article marketing blog You can instantly download over 400,000 of good quality plr articles on over 3000+ niche topics that you might edit and make use of as you wish. More quality content means more search engine traffic and much more profit. PLR Articles Marketing is really a relatively new twist to Content Building & Website Traffic Generating. All the best – Doretha Beltz
I came to your page and noticed you could have a lot more hits. I have found that the key to running a website is making sure the visitors you are getting are interested in your subject matter. There is a company that you can get visitors from and they let you try their service for free. I managed to get over 300 targeted visitors to day to my site. Check it out here: http://posco.com.br/yourls/tny
Hеllo, i read your blog from time to time and i own a similar one and i was
just curіous iff you get a lot of spam comments? If so how do
you reducе it, any plugin or anything you can suggest?
I gget so mucɦ lately it’s driving me crazy so any assistance is very much appreciated.
Howdy! I could have sworn I’ve visited this web site before but after going through a few of the posts I realized it’s new to me. Nonetheless, I’m definitely delighted I came across it and I’ll be bookmarking it and checking back regularly!
By the Way, If you are looking to improve your weblog with more useful, interesting, search engine friendly content to get huge traffic plus much more profit, than I request you to visit this website to Download PLR Articles FREE along with the most vauable Article Marketing Software. Best wishes – Billie
Hey there, I am so glad I found your web site. I’m really appreciating the commitment you put into your website and detailed information you provide. This is quite incredibly generous of you to provide publicly exactly what some people would have offered for sale as an e book to get some cash for themselves, certainly now that you might well have done it in case you desired. Please let me know if you’re looking for a writer for your site. You have some really good articles and I think I would be a good asset. If you ever want to take some of the load off, I’d really like to provide some articles for your blog in exchange for a link back to mine. Please send me an e-mail if interested. Many thanks!
You need targeted visitors for your website so why not get some for free? There is a VERY POWERFUL and POPULAR company out there who now lets you try their website traffic service for 7 days free of charge. I am so glad they opened their traffic system back up to the public! Check it out here: http://axr.be/17r1
Having read this I thought it was very enlightening. I appreciate you finding the time and effort to put this information together. I once again find myself spending a significant amount of time both reading and leaving comments. But so what, it was still worthwhile!